Translate this page into:
Dental Implant Treatment Planning on “Implant Design” – Review of Literature

* Corresponding author: Dr. Subia Ekram, Department of Oral and Maxillofacial Surgery and Oral Implantology, Dental Institute, Rajendra Institute of Medical Sciences, Ranchi, India. subia.ekram786@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Noorani MK, Ekram S. Dental Implant Treatment Planning on “Implant Design” – Review of Literature. Dent J Indira Gandhi Inst Med Sci. 2023;2:116–22. doi: 10.25259/DJIGIMS_3_2023.
Abstract
Dental implants encounter diverse forces of different magnitude and directions during mastication. Long-term success relies on factors like implant design, stability and materials used. Dental implants effectively prevent bone loss, address edentulous conditions and offer improved replacement options. Various factors can lead to failure, and some dentists may lack the necessary qualifications for success. The purpose of this article is to help learners make informed decisions when selecting the appropriate implant design
Keywords
Dental implant
Implant design
Osseointegration
BACKGROUND
Dentistry has seen remarkable progress in restorative materials, techniques and strategies, ensuring effective, long-term management of tooth loss. The ultimate goal of dental implant therapy is to satisfy the patient’s desire to replace one or more missing teeth in an esthetic and functional manner with long-term success.[1] Through scientifically proven approaches, dental patients now have access to excellent esthetic and functional options for tooth replacement. Individuals with partially missing teeth can opt for implant-supported crowns, which offer comparable function and esthetics to their natural teeth. Successful dental implant treatment requires careful treatment planning, meticulous surgical technique, and precise prosthetic restoration. A typical implant team consists of a restorative dentist, a properly trained and experienced surgeon and a dental laboratory technician who work together.
Osseointegration plays a crucial role in the success of dental implant therapy as it enables a reliable and predictable replacement of missing teeth. In histological terms, osseointegration refers to the direct and functional connection between the implant’s load-bearing surface and organized living bone, without any intervening soft tissue. The features of the design of dental implants are one of the most critical factors that have an effect on primary stability, and on the implant’s ability to sustain loading after osseointegration.[2]
INFLUENCE OF IMPLANT MATERIAL ON OSSEOINTEGRATION
There are no excellent scientific studies from which it can be deduced as to which implant design or surfaces are superior. Most of the time, implant success will depend on treatment planning, surgical skills, prosthetic design and patient behavior rather than on the implant surface. In a retrospective analysis of 2,349 implants in 677 patients to identify risk factors associated with failures of Bicon implants using multivariate regression analysis, it was determined that implant surface was not a factor, but that failures were due to tobacco use, implant length, staging, well size and immediate implants.[3] Osseointegration per se is not linked to any particular surface characteristics, because a great number of different surfaces achieve clinical osseointegration. However, stronger or weaker bone responses may be related to the surface characteristics.[4]
The dental implant body can be characterized using five features: its shape, surface macrostructure, surface microstructure, length and diameter.
Implant design
Dental implants come in various designs as shown in Figure 1, including threaded and non-threaded, as well as cylindrical or “press-fit” types.[5] Implant design can increase surface area of support. A threaded design as shown in Figure 2 implant has 30%–200% greater surface area compared to a cylinder implant of the same size. Although more difficult to place, the threaded implant is strongly encouraged in poorer-density bone. Biomechanical aspects of thread designs also affect the total increase in the surface area (i.e., thread pitch, shape, and depth).[6]

- Different Implant Design. (a) - Non-threaded implant of Endopore system with hexagonal internal connection. (b) - Threaded implant with square threads and hexagonal external connection. (c) - Parallel body design with self-tapping thread design from Alpha Bio. (d) - Tapered body with variable thread design. (e) - Tapered implant from Nobel Biocare with triangular internal connection. (f) - Tapered internal connection implant. (g) - Taper internal connection. (h) - Soft tissue–level single-stage implant from Straumann. (i) - White sky zirconium implant. (j) - One-piece implant
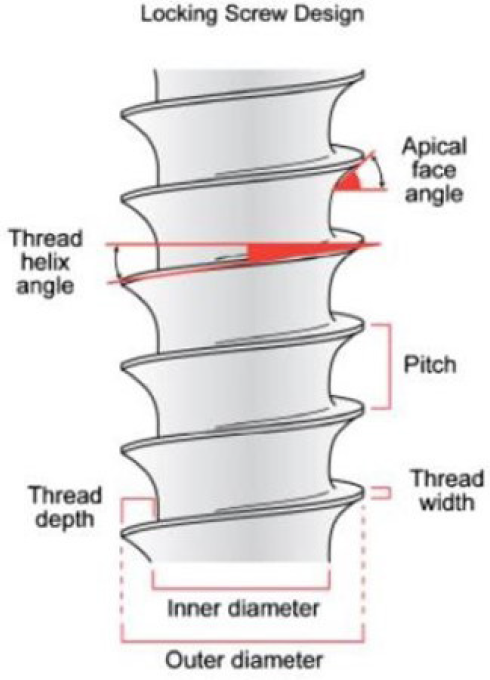
- Implant Thread Design.
Studies have demonstrated that introducing a roughened surface or applying hydroxyapatite coating on implants can accelerate osseous adaptation and enhance initial rigid fixation. In addition, an increase of surface-to-bone contact and amount of lamellar bone, and the relatively greater strength of the coronal bone around the roughened-surface implants occur when compared with machined or smooth titanium implants.[7,8] Therefore, utilizing coatings or roughened surfaces on implant bodies is advised for cases with compromised bone density, categorized as D3 or D4.
Discussion
The primary objective of implant design is to enhance surgical success rates and minimize complications related to plaque formation following treatment. Surgical success rate can depend upon when proper osseointegration takes place. The rationale behind macro and micro features in implant design is that the bone is stronger when loaded in compression, contrary to when subjected to tensile force and loaded in shear. Thus, when an implant is placed, it should be attempted to increase the compressive force exerted.[9,10] It is often supported by Wolff’s law that asserts that nature prioritizes efficiency in bone structure and tends to remove bone that isn’t optimally utilized, is substantiated”.[11]
Implant shape can be of different types such as tapered or threaded and cylindrical or smooth cylindrical. Cylindrical implants can be placed in anterior and posterior teeth regions, but tapered implants can be placed only in the anterior region. Smooth-sided cylindrical implants offer simplicity during implant placement, but they also result in increased shear conditions.
Implant thread is incorporated into implants to improve the initial stability[12,13] enlarging implant surface area and distributing stress favorably.[14,15] Kohn et al. [16] demonstrated the presence of a bone bridge from the depth of “1” thread to another. After subjecting the implants to lateral loading, it was observed that strain concentration occurred primarily at the point where bone contacts the crest of the thread, with a gradual decrease in strain towards the root of the thread.
Thread shape is set by the thread thickness and thread face angle. Knefel[17] investigated five different thread profiles and found the most favorable stress distribution to be demonstrated by an “asymmetric thread,” the profile of which varied along the length of an implant. Misch et al. [9] suggested that “V” shape (30° angle) generates higher shear force than reverse buttress thread (15° angle). Both types of threads are shown to get forces that can result in defect formation.[18]
Pitch refers to the distance between the centers of two consecutive threads measured parallel to the screw’s axis. This parameter is crucial as it influences the available surface area for bone-to-implant contact (BIC). A lower pitch leads to an increased number of threads, providing a larger BIC and surface area, resulting in a more favorable stress distribution. According to Kong et al.,[16] 0.8 mm is the optimal thread pitch for primary stability and optimum stress production on cylindrical implants with V-shaped threads. Thread geometry encompasses factors such as thread pitch, depth and shape, which contribute to how stress is distributed between the implant and the surrounding bone. This stress distribution is noticeable during the initial implant placement, the healing process and when the implant is under load.
Threads are used to maximize initial contact, improve initial stability as shown in Figure 3, enlarge implant area,[19] and they favor the dissipation of interfacial stress.[20] Thread depth, thread thickness, thread face angle, thread pitch and thread helix angle are some of the varying geometric patterns that determine the functional thread surface and affect the biomechanical load distribution of the implant.[21] The impact of threads becomes clear when considering that higher number of threads and deeper threads result in an increased functional surface area.
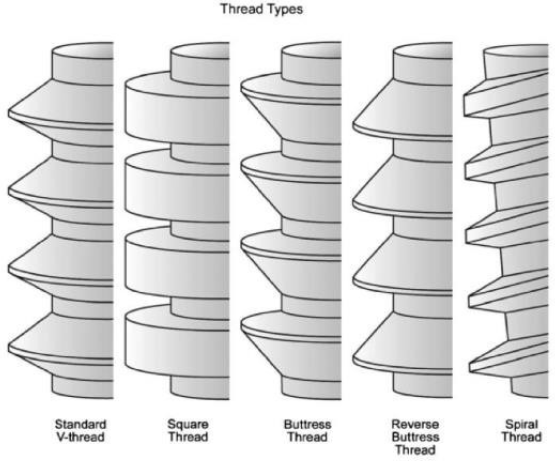
- Thread type.
The Implant Diameter is measured from the crest of the widest thread to the identical point on the alternative side of the implant.[22] According to the diameter, implants would also be classified as mini when the diameter is £2.7 mm, narrow when the diameter is >2.7 mm but £3.75 mm, regular when it ranges from 3.75 to 5 mm, and wide when the diameter is >5 mm. An increase in the diameter of an implant is associated with an increase in its surface area. For instance, increasing the diameter in a 3 mm implant by 1 mm increases the surface area by 35% over the same length.[23] Also, a 3.75 × 10 mm implant has 61% less surface area than a 6 mm diameter implant of the same length [Figure 4 and 5]. Typically, the diameter of the roots is measured approximately 2 mm below the cementoenamel junction [Figure 4]. To achieve an ideal emergence profile for the restoration, the implant platform is commonly positioned around 2 mm below the cementoenamel of adjacent teeth. Placing the implant too deep below the bone crest may result in increased crown height, posing a risk of mechanical failure in implant components and potentially affecting the esthetic results. When the implant is placed more superficially, restoration may be deemed impossible, and esthetic treatment outcome is also compromised.[24] It is important to differentiate between the implant diameter and platform diameter since they may not be the same. The implant platform refers to the portion of the implant that connects to the prosthetic (abutment) counterpart.
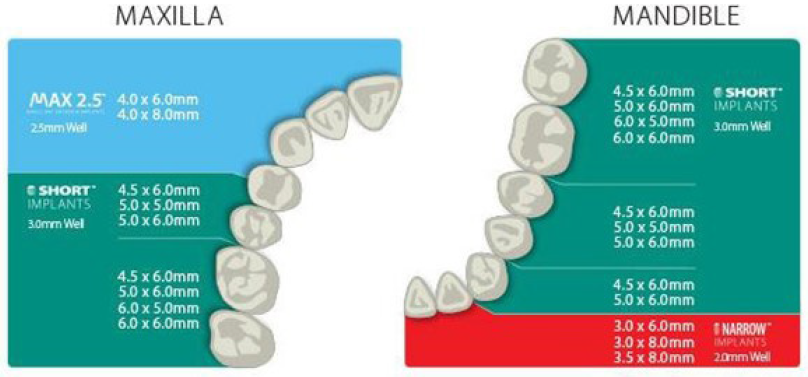
- Implant size chart.
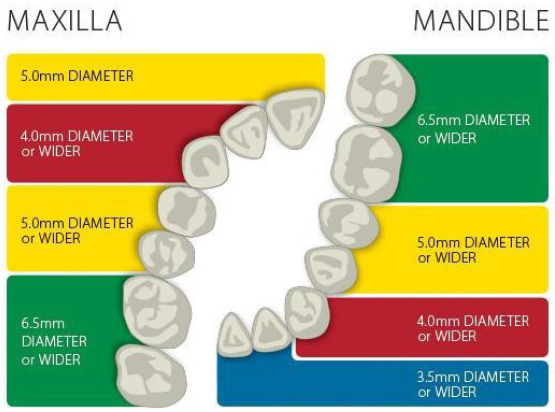
- Abutment Diameter Selection Guide for Non-Shouldered Abutments. *The chart above contains recommendations only. Actual clinical conditions and the clinician’s assessment of the patient should be the main criteria for choosing the size of the implant.
According to Albrektsson et al.,[25] factors affecting implant osseointegration are surgical technique host bed, implant design, implant surface material biocompatibility, and loading conditions.[25] Implant designs with internal connections exhibit approximately 0.2–0.3 mm of bone loss, whereas external connections show an average bone loss of up to 1 mm.
Multiple factors, however, influence the choice of the radiographic techniques used for any particular case [Table 1 and 2]. Factors such as cost, availability, radiation exposure and the type of case must be weighed against the accuracy of identifying important anatomic structures within a given bone volume, and the ability to perform the surgical placement without injury to these structures.[26]
| · Identifying the position of important structures, such as the mandibular canal, anterior loop and extension of the mandibular canal, mental foramen, maxillary sinus (floor, septa, walls and any pathologic features), nasal cavity and incisive foramen. |
| · Assessing bone height. |
| · Examining the proximity and angulation of the existing teeth in relation to roots. |
| · Evaluating the cortical bone. |
| · Analyzing bone density and trabeculation. |
| · Identifying any pathologic features, including abscesses, cysts and tumors. |
| · Detecting the presence of anatomic variants, such as incomplete healing of extraction sites and impacted teeth. |
| · Examining cross-sectional topography and angulation using CT and CBCT scans. |
| · Evaluating sinus health using CT and CBCT scans. |
| · Determining the skeletal occlusal classification through lateral cephalometric images |
| · Identifying the position of important structures, such as the mandibular canal, anterior loop and extension of the mandibular canal, mental foramen, maxillary sinus (floor, septa, walls and any pathologic features), nasal cavity and incisive foramen. |
| · Assessing bone height. |
| · Examining the proximity and angulation of existing teeth in relation to roots. |
| · Evaluating the cortical bone. |
| · Analyzing bone density and trabeculation. |
| · Identifying any pathologic features, including abscesses, cysts and tumors. |
CT: Computed tomography, CBCT: Cone beam computed tomography
| · A minimum of 1 mm below the floor of the maxillary and nasal sinuses. |
| · Avoidance of the incisive canal for maxillary midline implant placement. |
| · Five millimeters anterior to the mental foramen. |
| · Two millimeters above the mandibular canal. |
| · Three millimeters from adjacent implants. |
| · One and a half millimeters from the roots of adjacent teeth. |
A comparison of implant stability readings by Bailleri and colleagues[27] failed to demonstrate a right-way relationship between implant length and primary stability. Their results indicated that a short implant could be as stable as a long implant. Primary implant stability plays a vital role in successful osseointegration. In addition to length and width, thread shape and thread details also play a dominant role in local stress patterns at the bone–implant interface. Factors like thread pitch, depth and width impact the distribution of stress forces around the implant–bone contact area. The primary objective of thread design should be to reduce or prevent stress peaks during loading. The ultimate goal of the thread design should be to avoid or minimize stress peaks under loading. In this regard, thread depths seemed to play a very significant role on implant stability in both trabecular and cortical bone, whereas variations of pitch (the distance from the center of the thread to the center of the next thread) and the thread face angle (the angle between the face of a thread and a plane perpendicular to the long axis of the implant) do not influence implant stability in trabecular bone.[28] Chung and colleagues[29] found that implants with a pitch distance of 0.6 mm had more crestal cortical bone loss than implants with 0.5-mm pitch. These Implant Design and Osseointegration[30] are suggestive of the pitch being decreased, the surface area being increased, leading to a more favorable stress distribution. In situations involving poor bone quality, areas subjected to high occlusal forces and short implants, it is recommended to use implants with a greater number of threads. However, from a surgical perspective, fewer threads on the implant facilitate easier and faster insertion. Misch and colleagues[9] stated that “the deeper the threads, the broader the surface area of the implant,” which is a design that is very advantageous in softer bone and higher occlusal force areas because of a higher functional surface area in contact with the bone itself. In summary, implant threads should provide enhanced stability and a larger surface contact area for the implant.
Complication associated with dental implants
Commonly occurring complications can be classified as follows:[31]
According to Pjetursson et al. in 2008 (A 5-year cumulative complication rate)
-
Fracture of prosthesis: 13.2%
-
Peri-implantitis or peri-implant mucositis: 8.6%
-
Loss of the screw access restoration: 8.2%
-
Abutment screw loosening: 5.8%
-
Abutment screw fracture: 1.5%
-
Fracture of implants: 0.4%
Complications can be classified as follows:
-
According to Carranza et al.[32]
-
Surgical Complication
-
Hemorrhage and Hematoma
-
Neurosensory disturbances
-
Damage to adjacent teeth
-
Implant malposition
-
Related to sinus lift procedures
-
-
Biological Complication
-
Inflammation
-
Peri-implantitis and bone loss
-
Implant mobility
-
Dehiscence and recession
-
-
Mechanical/Technical Complication
-
Screw loosening and fracture
-
Implant failure
-
Fracture of framework or restorative material
-
Cement failure
-
-
Esthetic/Phonetic Complication
-
Esthetic complications
-
Phonetic complications
-
-
-
According to Misch and Wang (2008)
-
Early Stage Complications
Infection
Oedema
Ecchymosis
Bleeding
Flap dehiscence
-
Late-Stage Complications
Perforation of mucoperiosteum
Mandibular fractures
Failed osseointegration
Maxillary sinusitis
Bony defects
Periapical implant lesion
-
-
-
Intra-operative complications
-
Endosteal implants
-
Oversized osteotomy
-
Perforation of cortical plates
-
Fracture of cortical plates
-
Broken burs
-
-
Subperiosteal implants
-
Loss of anesthesia
-
Inability to make an accurate impression.
-
Antral perforation
-
Injury of the mental or infraorbital nerve
-
-
-
Short-Term Complications
-
Endosteal implants
-
Dehiscent wounds
-
Dehiscent implants
-
Radiolucency
-
Pterygomandibular raphe
-
-
Subperiosteal implants
-
Pterygomandibular raphe
-
Scar contraction
-
Post-operative infection
-
-
-
Long-term complications
-
Endosteal implants
-
Broken prosthetic inserts
-
Screw fracture
-
The inaccurate fit of castings
-
-
Subperiosteal implants
-
Bone resorption
-
Broken abutments
-
Recurrent peri-cervical granuloma
-
-
-
| Implant complication | ||
|---|---|---|
| First stage (during surgery) | Second stage (abutment connection) | Third stage (prosthetic phase) |
| Hemorrhage during drilling | Sensitivity | Loosening of abutment screws |
| Implant mobility after placement | Mobile implant (slight) and painful |
Fracture
|
| Exposed implant threads | Difficult in insertion | Bleeding on probing |
| Post-operative pain | Formation of granulation tissue around implant | Implant fracture |
| Lower lip insensitivity | Bone loss around implant | |
| Exposed cover screw after few days | ||
| Abscess around cover screw | ||
CONCLUSION
Determining success in implant dentistry remains a multifaceted challenge. There is no universally agreed-upon definition of clinical success for either natural teeth or dental implants. Both teeth and implants do not provide a clear-cut diagnosis of total health or failure. Even a tooth with periodontal pocket depths of 5 mm may be considered a “success” with appropriate therapy. Failure is generally more straightforward to identify, but if a dental unit does not meet the criteria for failure, it doesn’t necessarily mean it is successful. The primary criteria for evaluating implant quality are the presence of pain and mobility. If either of these factors are present, it significantly compromises the implant, and removal is often necessary.
According to Misch [9] criteria, for success in implant dentistry the following are necessary:
-
Pain
-
Rigid fixation
-
Probing depth
-
Bone loss
-
Bleeding index
-
Peri-implant disease
-
Percussion
-
Radiographic evaluation
Declaration of patients consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Introduction to Implant Dentistry A student Guide (2nd ed. Suppl.). USA: Elizabeth Perill, Michele Willmunder; 2017.
- Implant surface material, design, and osseointegration. Dental Clinics of North America.. 2015;59:505-20.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for dental implant failure: A strategy for the analysis of clustered failure-time observations. J Dent Res. 2002;81:572-7.
- [CrossRef] [PubMed] [Google Scholar]
- On implant surfaces: A review of the current knowledge and opinions. Int J Oral Maxillofac Implants. 2010;25:63-74.
- [PubMed] [Google Scholar]
- Chapter 22 – Maxillary posterior edentulism: treatment options for fixed prostheses. (2015).
- Functional surface area: thread for parameter optimization for implant body design. Compend Contin Educ Dent. 1998;19:4-9.
- [PubMed] [Google Scholar]
- Bone-to-implant apposition with machined and MTX microtextured implant surfaces in human sinus graft. Int J Periodontics Restorative Dent. 2003;23:427-37.
- [PubMed] [Google Scholar]
- Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J Biomed Mater Res. 2004;71:108-17.
- [CrossRef] [PubMed] [Google Scholar]
- Scientific Rationale for Dental Implant Design. In: Contemporary Implant Dentistry, (Misch CE) (3rd ed). Mosby Elsevier: St. Louis, Missouri; 2008. p. :200-229.
- [Google Scholar]
- The effect of thread pattern upon implant osseointegration. Clin. Oral Impl. Res. 2010;21:129-36.
- [CrossRef] [PubMed] [Google Scholar]
- Skeletal structural adaptations to mechanical usage (SATMU): Redefining Wolff law: the bone modeling problem. Aanat Rec. 1990;226:403-4.
- [CrossRef] [PubMed] [Google Scholar]
- Holding power of different screws in the femoral head. A study in human cadaver hips. Acta Orthop Scand. 1984;55:349-51.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of implant diameter on integration of screw implants. An experimental study in rabbits. Int J Oral Maxillofac Surg. 1997;26:141-8.
- [CrossRef] [PubMed] [Google Scholar]
- Biomaterials and biomechanics in dental implant design. Int J Oral Maxillofac Implants. 1988;3:85-97.
- [PubMed] [Google Scholar]
- Numerical investigations of the influence of implant shape on stress distribution in the jaw bone. Int J Oral Maxillofac Implants. 1989;4:333-40.
- [PubMed] [Google Scholar]
- Optimized thread pitch design and stress analysis of the cylinder screwed dental implant. Hua Xi Kou Qiang Yi Xue Za Zhi. 2006;24:509-12–15.
- [PubMed] [Google Scholar]
- Dreidimensionale spannungsoptische Untersuchungen verscheidener Schraubenprofile bei zahnarztlichen Implantaten. Dissertation, Ludwig-Maximilians-Universitat, Munchen; 1989
- The implant thread as a retention element in cortical bone: The effect of thread size and thread profile: A finite element study. J Biomechanic. 2003;36:1247-58.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of variations in implant diameters: A 3- to 5-year retrospective clinical report. Int J Oral Maxillofac Implants. 1999;14:173-80.
- [PubMed] [Google Scholar]
- Biomechanical considerations in dental implant design. Int J Oral Implantol. 1988;5:31-4.
- [PubMed] [Google Scholar]
- Contemporary Implant Dentistry (2nd Edition). Mosby: St. Louis, Missouri; 1993.
- Effect of implant size and shape on implant success rate: A literature review. J Prosthet Dent. 2005;94:377−81.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical properties of trabecular bone in the human mandible: Implications for dental implant treatment planning and surgical placement. J Oral Maxillofac Surg. 1999;57:700−6.
- [CrossRef] [PubMed] [Google Scholar]
- Dental Implant Restoration: Principles and Procedures (1st edition). New Malden, UK: Quintessence Publishing; 2001.
- Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981a;52:155-70.
- [CrossRef] [PubMed] [Google Scholar]
- Contemporary Oral and Maxillofacial Surgery (6 edition). St. Louis, MO: Elsevier; 2014.
- Stability measurements of osseointegrated implants using Osstell in partially edentulous jaws after 1 year of loading. Clin Implant Dent Relat Res. 2002;4:128-32.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of thread features in osseointegrated titanium implants using a statistics-based finite element method. Dent Mater. 2012;28:919-27.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of implant geometry and surface treatment on osseointegration after functional loading: A dog study. J Oral Rehabil. 2008;35:229-36.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of plasma-sprayed hydroxyapatite coating on the osteoconductivity of commercially pure titanium implants. Int J Oral Maxillofac Implants. 2000;15:483-90.
- [PubMed] [Google Scholar]
- A systematic review of the 5‐year survival and complication rates of implant‐supported single crowns. Clin Oral Implants Res. 2008;19:119-30.
- [CrossRef] [PubMed] [Google Scholar]
- Carranza’s Clinical Periodontology. Elsevier Health Sciences; 2011.
- Implant reversible complications: Classification and treatments. Implant Dent. 2005;14:211-20.
- [CrossRef] [PubMed] [Google Scholar]
- Blood loss during periodontal flap surgery. J Periodontol. 1977;48:693-8.
- [CrossRef] [PubMed] [Google Scholar]







