Translate this page into:
Leukoplakia with Diverse Grades of Epithelial Dysplasia – A Case Series and Review of Literature with Updated Management Protocol

*Corresponding author: K.V. Sai Charan Department of Oral Medicine and Radiology, Sathyabama Dental College and Hospital, Semmanjeri, Chennai, India. kvss1996@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: KVS Charan. Leukoplakia with Diverse Grades of Epithelial Dysplasia – A Case Series and Review of Literature with Updated Management Protocol. Dent J Indira Gandhi Int Med Sci. 2024;3:44–50. doi: 10.25259/DJIGIMS_23_2023
Abstract
Oral leukoplakia is considered the most potentially malignant disorder of the oral cavity, with a high risk of malignant transformation. Based on the clinical manifestation, it has been classified as a homogeneous and non-homogeneous variant. It has been considered predominantly a white lesion of the oral cavity that cannot be classified under any other definable lesions. Leukoplakia is a term used to describe the lesion clinically. There are various components responsible for the threatening change of oral leukoplakia. The clinical diagnosis of leukoplakia is confirmed by histopathology, which commonly indicates intense epithelial dysplasia. This manuscript describes a series of cases diagnosed clinically as leukoplakia and their grades of epithelial dysplasia, a Review of literature about etiology and the key pathogenesis behind it, risk determinants for malignant transformation, chair side investigation for epithelial dysplasia, and updated management protocol.
Keywords
Leukoplakia
Epithelial dysplasia
Biopsy
Malignant transformation
Risk determinants
INTRODUCTION
Leukoplakia is a term that ensued from Greek literature (Leucos-white, Plakia-patch). This terminology was in parlance since, the latter half of the 19th century.[1] Amongst the potentially malignant disorders (PMDs) of the oral cavity, oral leukoplakia (OL) is the most typically encountered clinical entity in dental practice. Numerous definitions have been proposed to describe the OL for five decades.[2] Nevertheless, there is still a misperception and indistinctness in uniform reporting of this lesion. In 2017, Shanbhag VL defined leukoplakia as “a predominantly white, irreversible, non-scrapable lesion of the oral mucosa that cannot be characterized clinically or histopathologically as any other lesion or disease and has increased risk of cancer occurrence than its normal counterpart and is usually associated with consumption of tobacco, betel quid, and alcohol, but otherwise can be of idiopathic in nature.”[3] In India, the prevalence of leukoplakia is 0.2-4.9%.[4] The major challenge confronted by Oral Medicine specialists is to evaluate the status of risk and elucidate the potentially malignant status of OL in a clinical setup. This article aims to discuss the series of cases diagnosed as leukoplakia clinically and their dysplastic changes. Review of literature with regard to etiology and their key mechanism, risk determinants for malignant transformation, chair side investigation for epithelial dysplasia, and updated management protocol.
CASE REPORT 1
A 40-year-old male came to our department with the chief complaint of a whitish patch in his mouth for the past two months. He noticed the lesion while brushing his teeth, and the patient experienced a burning sensation while consuming hot and spicy food. On explicating the habit history, the patient had the habit of chewing tobacco for the past 20 years (pouches the tobacco quid in the right and left buccal vestibule 4-5 times a day). Additionally he also had a history of cigarette smoking for the past the 10 years (10 cigarette/day) and a history of alcohol consumption for the past 17 years (375 ml/day). The patient had no contributing medical history. On extraoral examination, no significant abnormalities were detected. On intra-oral examination the erythematous patch with grayish white specks was seen on the right and left buccal mucosa; on both sides it was approximately measuring about 5 cm × 3 cm seen extending from the commissure of the lip to the second molar region posteriorly. The lesion was irregular in shape, and the surrounding areas appeared normal. On palpation, the lesion was non-tender, granular in consistency, and non-scrapable with no signs of induration. The provisional diagnosis of speckled leukoplakia of right and left buccal mucosa was made based on clinical presentation and history. The patient was subjected to blood investigations, including complete blood count (CBC), bleeding time (BT) and clotting time (CT). His blood investigations were normal, and the incisional biopsy of the lesion from the right buccal mucosa was done and sent for histopathological examination [Figure 1]. The histopathological findings were, surface epithelium exhibited broad bullous rete ridges, basilar hyperplasia, and multiple high-level basilar cells. Fibrosis and dense mild mixed inflammatory cell infiltrate and foci of dense chronic inflammatory infiltrate in connective tissue, are suggestive of mild epithelial dysplasia [Figure 2]. Habit cessation counseling was given ,and antioxidants (Tab. Antoxid) were prescribed for one month. Additionally, topical 0.05% of tretinoin was prescribed and advised to apply once daily in bilateral buccal mucosa for two weeks.
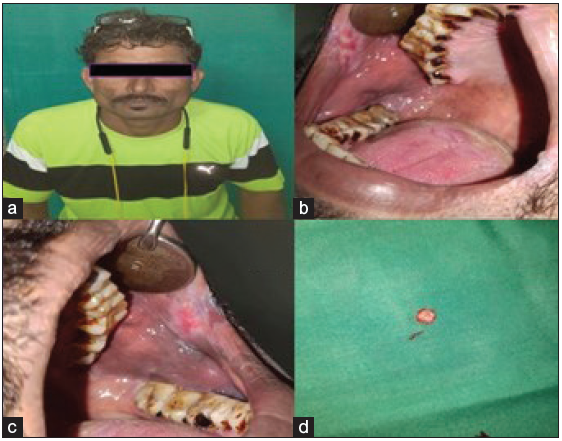
- (a) Patient straight profile, (b & c) Erythematous patch with grayish white specks seen on the right and left buccal mucosa, (d) Biopsied tissue.
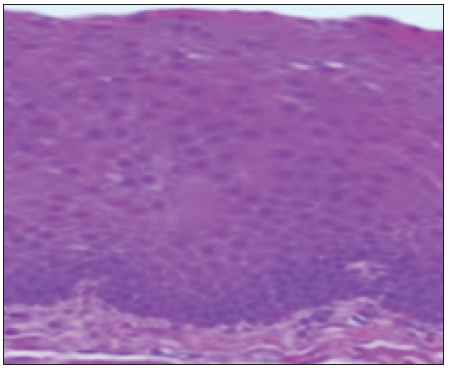
- Histopathology picture.
CASE REPORT 2
A 54-year-old male came to our department with the chief complaint of a grayish-white patch on the left side of the mouth, which he had noticed incidentally while brushing his tooth. The patient experienced a burning sensation while consuming spicy food. On explicating the habit history, the patient has had the habit of smoking for 15 years (bidi 10–15/day) and also gives a history of alcohol consumption for the past 10 years (500 ml/day). The patient had no contributing medical history. On extraoral examination, no significant abnormalities were detected. On intra-oral examination, the left buccal mucosa revealed an elevated grayish-white hyperkeratotic patch which was seen extending from the retro-commissure of the lip to the retromolar pad region posteriorly. The borders are well-defined with surrounding erythematous mucosa. The surface of the lesion appeared wrinkled and corrugated. On palpation, the lesion was leathery in consistency, non-scrapable, and non-tender with no signs of induration. The provisional diagnosis of homogeneous leukoplakia was made. The conditions like frictional keratosis, leukoedema, and chronic hyperplastic candidiasis were considered differential diagnoses. The patient was subjected to blood investigations, including CBC (complete blood count), BT (bleeding time), and CT (clotting time). His blood investigations were normal, and the incisional biopsy of the lesion from the left buccal mucosa was done and sent for histopathological examination [Figure 3]. The histopathological findings were para-keratinized stratified squamous surface epithelium in association with a fibrovascular connective tissue exhibiting mild diffuse chronic inflammatory cell infiltrate, the surface epithelium shows elongated bulbous rete ridges, basilar hyperchromatism, and pilocytic changes. Suggestive of mild epithelial dysplasia [Figure 4]. Habit cessation counseling was given, and antioxidants were prescribed for one month.;In addition, topical bleomycin 1% was prescribed, and advised to apply once daily in the left buccal mucosa for 14 days.
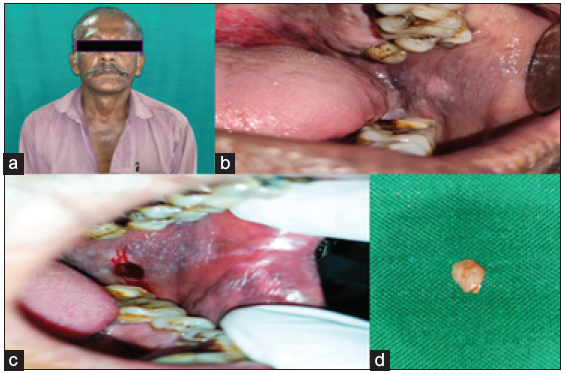
- (a) patient straight profile, (b) elevated grayish white hyperkeratotic patch, which was seen extending 1 cm behind the retro-commissure of the lip anteriorly to the retromolar pad region posteriorly, (c) biopsy site, (d) Biopsied tissue.
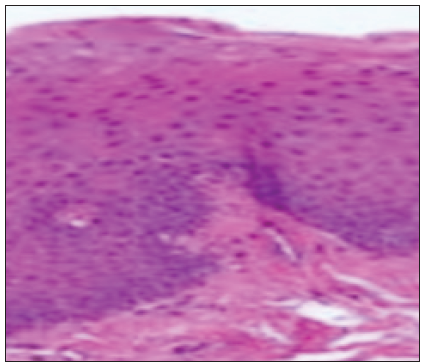
- Histopathology picture.
CASE REPORT 3
A 39-year-old male came to our department with the chief complaint of the discoloration of mucosa in the oral cavity for the past three months, and he incidentally noted the color change while brushing his tooth. On elucidating the habit history, he has had the habit of cigarette smoking for the past 20 years (6 cigarettes/day). The patient had no contributing medical history. On extraoral examination, no significant abnormalities were detected. On intra-oral examination, a grayish-white raised patch is evident on the right buccal mucosa, roughly measuring about 4 × 5 cm, seen extending from the retro commissure of the lips to the retromolar pad region at the line of occlusion. The borders are irregular, and the surface appears wrinkled and corrugated, imparting a cracked mud appearance. On palpation, the inspectory findings were confirmed, and the lesion was non-tender and non-scrapable with no signs of induration [Figure 5]. The provisional diagnosis of homogeneous leukoplakia was based on clinical presentation and contributory habit history. The differential diagnosis of hyperplastic candidiasis and the lichenoid reaction was considered. The patient was subjected to blood investigations, including CBC, BT and CT. His blood investigations were normal, and the incisional biopsy of the lesion from the left buccal mucosa was done and sent for histopathological examination. The histopathological findings were hyperkeratinized stratified squamous epithelium exhibiting foci of flattened epithelial connective tissue interface, foci of pilocytic changes, diffuse subepithelial chronic inflammatory cell infiltrate, and melanin incontinence suggestive of mild epithelial dysplasia [Figure 6]. Habit cessation counseling was given, and antioxidants were prescribed for one month. Moreover, topical bleomycin 1% was prescribed, and advised to apply once daily in the left buccal mucosa for 14 days.
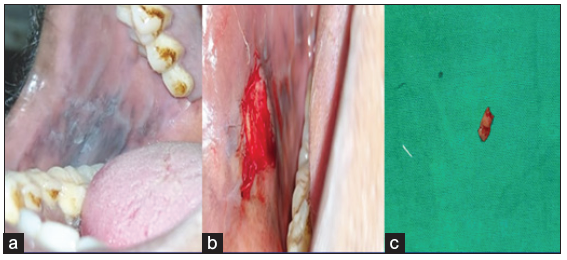
- (a) Elevated grayish white hyperkeratotic patch, (b) biopsy site, (c) Biopsied tissue.
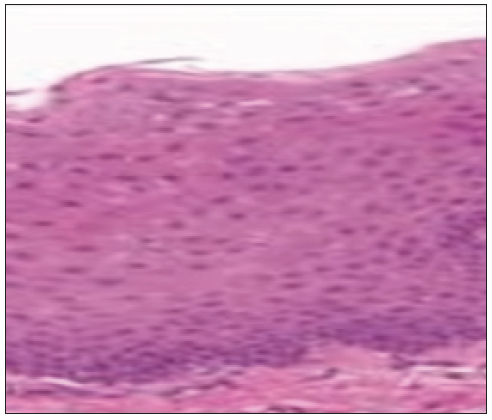
- Histopathology picture.
CASE REPORT 4
A 59-year-old male came to the department with the chief complaint of a a whitish patch in his mouth; he incidentally observed the color change while gargling. On elucidating the habit history, the patient has had a habit of smoking for the past 30 years (5/day) and alcohol consumption for the past 10 years. There was no contributing medical history. On extraoral examination, no significant abnormalities were detected. On intra-oral examination, the grayish-white raised patch was evident on the right buccal mucosa, roughly measuring about 2 × 1 cm, extending from the retro-commissure of the lip to the first pre-molar region. The borders are irregular, and the surface appears wrinkled and corrugated, imparting a cracked mud appearance. The inspection findings were confirmed on palpation, and the lesion was non-tender and non-scrapable with no signs of induration. The provisional diagnosis of leukoplakia was made. The differential diagnosis of frictional keratosis, hyperplastic candidiasis, and lichen planus (plaque Type) was considered. The patient was subjected to blood investigations, including CBC , BT, and CT. His blood investigations were normal, and the incisional biopsy of the lesion from the left buccal mucosa was done and sent for histopathological examination [Figure 7]. The histopathological findings revealed para-keratinized stratified squamous epithelium exhibiting acanthosis, intracellular edema, basilar hyperplasia, and bullous rete ridges. The fibrovascular connective tissue exhibits a mild focal aggregate of chronic inflammatory cell infiltrates. The surface epithelium shows broad pushing margins and few islands in the superficial connective tissue [Figure 8]. The final diagnosis is moderate epithelial dysplasia. There was a satisfactory healing on the biopsy site. Chair-side investigation of toluidine blue staining was performed in the right buccal mucosa to demarcate the areas of epithelial dysplasia, and a complete excision of the lesion was done. Habit cessation counseling was given. Antioxidants were prescribed for three months.
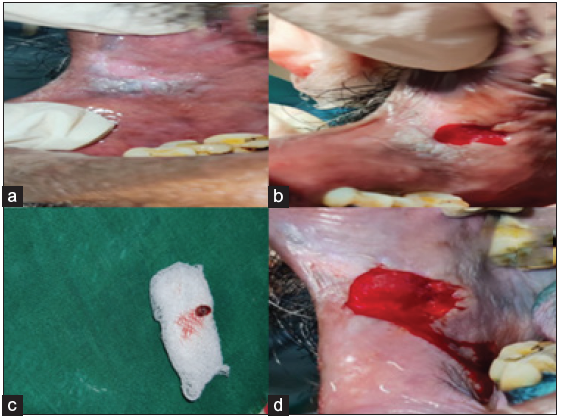
- (a) Elevated grayish white hyperkeratotic patch (b) Biopsy site, (c) Biopsied tissue, (d) Complete excision of the lesion following vital staining.
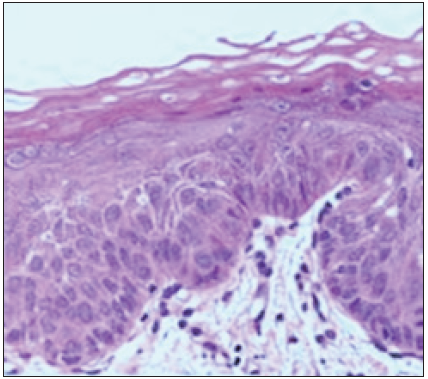
- Histopathological findings.
DISCUSSION
Tobacco in various formulations is considered the main causative factor for leukoplakia. Besides tobacco, alcohol is also found to be contributory. Though alcohol alone is not associated with the development of leukoplakia, it has nearly a synergistic effect in combination with tobacco in the development of this lesion.[4] The alcohol brings about dehydration of oral mucosa, and that, in turn, raises the temperature of the oral cavity. Thus, the oral mucosa becomes more susceptible to the carcinogenic effects of tobacco. Individuals who excessively use alcohol-based mouth rinses with a concentration of more than 25% develop pseudo-leukoplakia.[4]
Nutritional deficiency (Vitamin A, C, B-complex, E, β-carotene) can also be the cause of the occurrence of leukoplakia.[4]
Sanguinaria, the herbal extract from the flowering plant (Sanguinaria canadensis), an antiplaque agent in toothpaste and mouth rinses, is extremely used to treat gingivitis and is associated with leukoplakia. This form of leukoplakia is known as sanguinaria-associated keratosis, and it commonly occurs in the vestibule and alveolar mucosa of the maxilla [1,4]. Sanguinarine has a molecular structure comparable to polyaromatic hydrocarbon (chemical-carcinogens) and DNA intercalator. Sanguinarine also creates oxidative and endoplasmic reticulum stress, resulting in the unfolded protein response and the formation of 8-hydroxylamine, leading to the development of lesions.
Ultraviolet radiation has been implicated in leukoplakia of the lower vermillion border of the lip [4]
Other questionable etiological factors are:
Epstein-Barr Virus: Epstein Barr Virus (EBV) also produces oral leukoplakia, and it clinically appears as a thick whitish patch with a wrinkled, corrugated/hairy surface [1–4]
Candida albicans: Candida albicans commonly appear in histologic sections of leukoplakia. The commissure of the lips is the most commonly affected site.[4]
Syphilis and Human Papilloma Virus - particularly subtypes HPV-16 and HPV-18, have been identified in some oral leukoplakia. The role of this virus remains questionable.[3,4]
Whereas, in our case series, leukoplakia was strongly associated with smoking compared to other etiologies.
Leukoplakias are frequently diagnosed based on the patient’s history and clinical examination. It is mandatory to biopsy the lesions which are clinically assumed to be leukoplakias. The prime consequence of performing an incisional biopsy in such lesions is to perceive the presence or absence of dysplasia. Dysplasia is characterized by the atypical orientation of epithelial cells, cellular pleomorphism, and cellular atypia, suggestive of early malignancy.[1] Epithelial dysplasia is mild, moderate, and severe. 1) Mild dysplastic lesions are primarily confined to the lower one-third of the epithelium. 2) Moderate dysplastic lesions are cardinally confined to the lower two-thirds of the epithelium. 3) Severe dysplastic lesions involving the entire thickness of the epithelium but without breaching the basement layer of the epithelium.[4,5]
RISK DETERMINANTS FOR MALIGNANT TRANSFORMATION OF LEUKOPLAKIA
Gender Prediction: The female gender has been flagged as a risk factor for malignant transformation of Leukoplakia.[5] In India, the Malignant Transformation Rate of leukoplakia is higher in males than in females, probably because of the association with tobacco chewing and smoking.[5] Neither malignant transformation of Oral Leukoplakia concentrating exclusively on female patients nor the comparison of treatment outcomes between males and females has not been described yet.[6] The reason why the female gender is more susceptible to malignant transformation of leukoplakia remains an oxymoron.[2-6]
Duration: Neoplasia from the dysplastic lesions commonly progresses over (2–5 years) but can also advance later.[5]
Age: The malignant transformation rate of leukoplakia is known to increase with advancing age.[5,6] Nevertheless, consider a situation: a 60-year-old person with a history of smoking for 30 years diagnosed with leukoplakia with dysplasia and a 25-year-old person with a history of smoking for five years diagnosed with leukoplakia with dysplasia; in the given situation the younger aged person is at a high-risk category when compared to the old aged person.
Site: Leukoplakia on the floor of the mouth and lateral border of the tongue is flagged as a high risk of malignant transformation.[6] The biological reasons for the floor of the mouth to carry a higher risk have not been explored.[2] However, the possible reason could be gravity, a relatively lower thickness of mucosa in these regions compared to other areas of the oral cavity. Lymphatic drainage in the vicinity of these regions etc.
Habits: Commonly, Malignant transformation was found to be greater in smokers than non-smokers.[1-5] Nonetheless, the risk of malignant transformation in non-smokers is 7.1 times higher when compared to heavy smokers.[5,6]
Type: Speckled and erosive leukoplakia have the highest malignant transformation rate, Erythro-leukoplakia carries an average transformation potential of 28%. Proliferative verrucous leukoplakia showed a high degree of malignant transformation when compared to Homogeneous type 1%.[5,6]
OTHERS
CHAIR DIAGNOSTIC AIDS FOR THE DETECTION OF EPITHELIAL DYSPLASIA
Toluidine blue staining: Toluidine blue staining is a valuable adjunct to clinical examination in deciding the site of biopsy. It is a vital dye that stains the nucleic acids of the abnormal tissues. It has been used for the past two decades to aid in identifying mucosal abnormalities. Interpretation is based on the observed color; dark blue (Royal/Navy) is considered to be positive, whereas light blue/no color is interpreted as negative.
Light-based detection system: In recent years, tissue reflectance-based examination of oral mucosa has been adapted for use. The patient must be asked to first rinse his mouth with 1% acetic acid solution, which aids in the removal of debris and increased visibility of epithelial cell nuclei, followed by a direct visual examination of the oral cavity using a blue-white light source. Under blue-white illumination, normal epithelium appears lightly bluish, while abnormal epithelium appears distinctly white (acetowhite). The available light-based detection system includes Vizilite Plus & Microlux DL. Moreover, management of leukoplakia includes a multidisciplinary approach based on the presence of grades of epithelial dysplasia[7-14] the management protocol has been enlisted in Table 1.
| Leukoplakia without dysplasia | Leukoplakia with mild dysplasia | Leukoplakia with moderate and Severe dysplasia |
|---|---|---|
|
|
Complete excision of the lesion 1. Photodynamic therapy 2. Surgical excision with or without grafting 3. Electrocoagulation 4. Cryosurgery 5. Laser surgery Habit cessation counseling and regular follow-up |
Near future
Lab-on-chip technology is in its emerging phase in the detection of epithelial dysplasia and cancer using saliva as a sample. The basic mechanism of detection is by analyzing the biomarkers/nucleic acid detection by Real-time reverse transcriptase-polymerase chain reaction (RT-PCR), where all the components are installed in a chip. When these chips are approved and available in markets, they can be used as a chair-side investigation aid in detecting epithelial dysplasia.
Management of this lesion depends on the severity of dysplasia Management of leukoplakia includes multidisciplinary approach based on the presence of grades of epithelial dysplasia.[7-14] The management protocol has been enlisted in Table 1.
CONCLUSION
As leukoplakia shows higher malignant transformation, it is important for a clinician that once the diagnosis of leukoplakia is made, it is mandatory for a biopsy of the site to know the severity of dysplasia and manage it accordingly.
Acknowledgments
Nil
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
References
- Oral leukoplakia etiology, risk factors, molecular pathogenesis, prevention and treatment: A review. Int J Contemp Med Res. 2020;7:K1-K5. Available from: https://www.ijcmr.com/uploads/7/7/4/6/77464738/ijcmr_3275_v2.pdf [Last accessed on 2023 Dec 21]
- [Google Scholar]
- Malignant transformation of oral leukoplakia: A systematic review of observational studies. J Oral Pathol Med. 2016;45:155-66.
- [PubMed] [Google Scholar]
- New definition proposed for oral leukoplakia. Dent Res J. 2017;14:297-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Leuloplakia - review of a potentially malignant disorder. J Clin Diagn Res. 2014;8:ZE01-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J Oral Maxillofac Pathol. 2019;23:19-27.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A Retrospective Cohort Study of Oral Leukoplakia in Female Patients-Analysis of Risk Factors Related to Treatment Outcomes. Int J Environ Res Public Health. 2021;18:8319.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A review of the nonsurgical treatment of oral leukoplakia. Int J Dent. 2010;2010:186018.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of oral epithelial dysplasia: A review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:S19.e1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Prediction of malignant transformation in oral epithelial dysplasia using infrared absorbance spectra. PLOS ONE. 2022;17:e0266043.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of premalignant and malignant lesions by fluorescent light. J Int Soc Prev Community Dent. 2015;5:248-54.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Consensus guidelines on management of oral potentially malignant disorders. Indian J Cancer. 2022;59:442-53.
- [CrossRef] [PubMed] [Google Scholar]
- A case of tongue cancer manifesting from oral leukoplakia after long-term administration of pegylated liposomal doxorubicin. Oxf Med Case Reports. 2022;2022:omac042.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chemoprevention in oral leukoplakia: Challenges and current landscape. Front Oral Health. 2023;4:1191347.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Photodynamic therapy (PDT) for oral leukoplakia: A systematic review and meta-analysis of single-arm studies examining efficacy and subgroup analyses. BMC Oral Health. 2023;23:568.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







