Translate this page into:
APPLICATION OF PEEK: A FUTURE REVOLUTION IN PROSTHODONTICS
Corresponding Author: Dr. Kumari Sindhu Pravin
This article was originally published by Indira Gandhi Institute of Medical Science and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The aim of this study is to review polyether ether ketone (PEEK), its characteristics and use in prosthodontics. Polyetheretherketone (PEEK) is one of the most used semi-crystalline thermoplastic polymers in aeronautics and aerospace. This new material matches the technological advancements and patient's desires for a natural and aesthetic look, thanks to its strength, superior biocompatibility, low plaque affinity and aesthetics characteristics close to the desired natural dental structure. PEEK has good mechanical and electrical properties such as resistance to high temperature and resistance to hydrolysis. Satisfactory findings have also been observed when used in knee, hip, spine and other implants.
PEEK polymer is suitable to use in prosthodontics. PEEK can fulfil the long-term demand of an ideal multipurpose material for biomedical applications. However, there are not enough statements about complications, biofilm formation on PEEK surface and its resistance to compression. More research should be done to find out the results.
Keywords
Polyetheretherketone
PEEK
polymer
dental application
dental implant.
INTRODUCTION:
The most commonly used material for the fabrication of dentures is PMMA. Although PMMA is a non-metallic denture base material, there is still the possibility of toxic reactions or irritations for the wearer. Polysulfone (PSF), nylon and polycarbonate (PC) are suggested for patients who are allergic to acrylic1. However, shortcomings in some of their properties have limited their uses and it seems that as yet no material fully satisfies the ideal criteria for a denture base.
PEEK is quite a new material in prosthodontics. Comparing to the metals used in dentistry, PEEK is more aesthetic, stable, biocompatible, lighter and has reduced degree of discoloration. This makes it more attractive to patients with high aesthetic requirements1. PEEK has a modulus of elasticity close to the bone (3-4GPa), it can be used as an alternative to metal implants. PEEK is an inert material and its thermal properties remain stable in the human body2.
According to Katzer et al, PEEK and CFR-PEEK do not exhibit cytotoxic, mutagenic reaction. Similarly, PEEK does not show an allergic reaction. PEEK has high thermal degradation resistance, melting temperature is 334°C7.
Generally, the application of PEEK as unfilled polymer is the most popular form. However, for implant purposes much research has focused on PEEK biomaterial's biocompatibility with bioactive materials, including HA (hydroxyapatite) as a surface coating or as a composite filler3. Later still, the biomaterial studies indicated that PEEK and related composites can be engineered with a wide range of mechanical, physical and surface properties, depending on the form of implant application2.
In the late 1980s, the potential use of carbon fibre reinforcing PEEK attracted the interest of the medical implant community. Its versatile mechanical and physical properties as well as its outstanding biocompatibility can offer sustainability when used in combination with modern imaging technologies4. The CFR-PEEKs have demonstrated stiffness to closely match that of the cortical bone. Therefore, they have been widely used as alternatives to cobalt chromium and titanium alloys. Due to its excellent mechanical and aesthetic properties PEEK can also be used as framework for removable and fixed dental prosthesis, orthodontic wires5. This paper summarises the properties and application of PEEK for dental purposes.
HISTORY OF PEEK
Polyetheretherketone (PEEK) is a semi-crystalline linear polycyclic aromatic thermoplastic that was first developed by a group of English scientists in 1978. Late 1990s, PEEK became an important high performance thermoplastic candidate for replacing metal implant components, in vertebral surgery as a material of the interbody fusion cage3.
With the emergence of carbon fiber reinforced PEEK (CF/PEEK), this new composite material was exploited for fracture fixation and femoral prosthesis in artificial hip joints Later in 1998 it was proposed for biomedical application by Invibio Ltd (Thornton-Cleveleys, UK). In the same year Victrex PEEK business (Imperial Chemical Industry, London UK) launched PEEK- OPTIMA for long-term implantable applications4.
STRUCTURE AND SYNTHESIS OF PEEK
PEEK is a colorless organic thermoplastic polymer a member of PAEK family. PEEK as a dominant member of the PAEK family has a glassy state at room temperature; and at about 143 °C the glass transition temperature occurs, while at around 343 °C the crystalline melt transition of PEEK might take place. PEEK is a polycyclic, aromatic, thermoplastic polymer that is semi-crystalline and has a linear structure3. This material is obtained as a result of the binding of ketone and ether functional groups between phenyl rings [Figure - 1] and is an element which is tan-colored in its pure form.
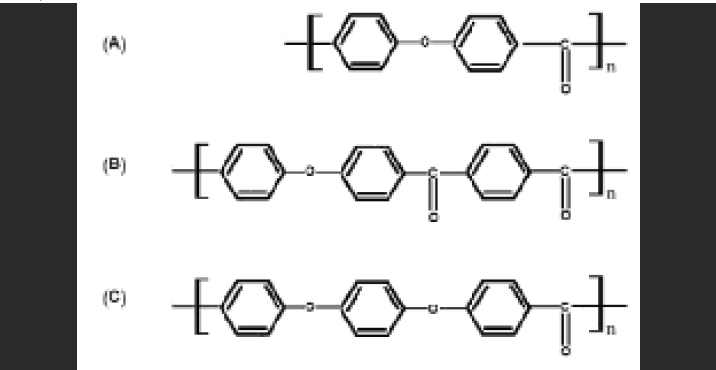
- The chemical structure of some PAEK homopolymers: (A) PEK; (B) PEKK; and (C) PEEK,
However, the nucleophilic route to PAEK polymers offers a simple pathway to PEEK polymer, with the addition of suitable solvents like benzophenone or diphenylsulfone, which should be inert and provide thermal stability to phenoxide species used in the synthesis of PAEK polymers. Hydroquinone and sodium or potassium carbonate was used to control the inherent instability of bisphenate to oxidation. Moreover, high temperatures above 300°C were needed to reach high molecular masses. Furthermore, a slight excess of difluorobenzophenone enhanced the control of molecular weight, leading to fluorine- terminated chains.
PROPERTIES OF PEEK
PEEK has excellent properties. It is the most biocompatible and has less young's modulus of elasticity (3 - 4 GPa) which is close to the human bone. Properties of PEEK is altered simply by addition of various materials, as elastic modulus can be increased by incorporation of carbon fibres. Branemark introduced the use of titanium and its alloys for dental implants8. This titanium alloys have significantly high elastic modulus resulting in intense stress shielding and non-success. The carbon reinforced PEEK has modulus of elasticity similar to dentin and cortical bone so the polymer could manifest lower stress shielding in contrast to titanium implants. Moreover, PEEK has excellent thermal properties, superior wear resistance, inertness, corrosion resistance, high strength and modulus of elasticity is analogous to enamel, dentin and cortical bone. Radiographic radiolucency and low density make it acceptable for medical applications. PEEK components are manufactured by rapid prototyping, CAD CAM (Computer-aided design and Computer-aided manufacturing) milling or by injection, extrusion and compression moulding techniques10.
Modification of Peek Implants
Surface modification methods have been developed to increase the bioactivity of the material. PEEK materials were classified depending on the size of the bioactive materials impregnated on it into two types:13
Conventional PEEK composites13
This includes carbon fibre-reinforced PEEK (CFR- PEEK), Glass fibre-reinforced] PEEK (GFR-PEEK), Hydroxyapatite/PEEK (HA/PEEK), Strontium-containing hydroxyapatite/PEEK (Sr- HA/PEEK)
Nanosized PEEK composites
This includes Nano- Ti02/PEEK, Nano-Fluorine apatite PEEK and Nano-hydroxyapatite PEEK
Surface treatment of PEEK to increase its bond strength to veneering composites:
Acid etching with 98% sulfuric acid for 1 min and careful rinsing with deionized water for 1 min.
Air abrasion with alumina with a mean particle size of 50 mm or 100 mm for 10s at a pressure of 2 bar and at a distance of 10 mm between the nozzle and the surface.
Silica coating for 10 s and subsequent application of silane for5 min.
Polyetheretherketone (PEEK) in Prosthodontics PEEK in Dental Implant
PEEK dental implants have not been extensively used clinically and therefore more studies are essential to identify their long-term efficacy in human subjects. Considering adequate biocompatibility, implant healing abutments can be constructed using PEEK18,19. there is no significant difference in bone resorption and soft tissue inflammation between PEEK and titanium abutment18. In addition, oral microbial flora attachment to PEEK abutments is equivalent to that of titanium, zirconia and polymethylmethacrylate abutments19. Titanium could be replaced by PEEK in constructing implant abutments, since the elastic modulus of bone and PEEK reduces stress shielding effects and encourages bone remodelling [Figure-2].
In a study by Cook et al, PEEK implants strengthened with carbon fiber (CFR-PEEK) and titanium covered CFR-PEEK implants were implanted to femurs and evaluated after 8 weeks. Similar bone-implant contact ratios were reported13. To increase cell attachment in the PEEK implant surface, studies have been made on hydroxyapatite (HA) coating. Promising results have been obtained from PEEK implants coated with HA in comparison with non-coated PEEK implants14. When current research is examined, it can be seen that there are still no long-term studies of the efficacy of this material on patients. Therefore, PEEK implants are not widely used clinically.
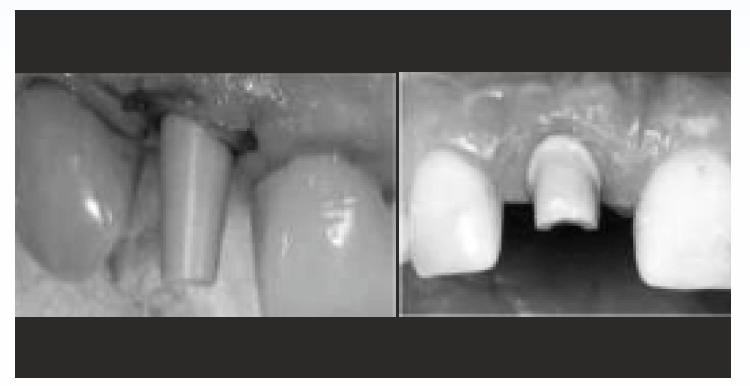
- PEEK as abutment
PEEK as abutment
PEEK in implant substructures
■ It can be applied in Dental implants as implant superstructure, abutment or body. There is current research on reinforcing PEEK material by additives and surface modifications. The tests applied have shown that PEEK material is resistant up to 1200N of chewing forces15.
■ Major benefit of PEEK as an implant material is its Youngs modulus which is close to human bone, thus it can generate favorable stress and deformations minimizing stress shielding effect and bone resorption. Unfilled PEEK exhibits an elastic modulus of 3-4Gpa15. The addition of reinforcing agents like carbon fibers increases elastic modulus of PEEK up to 18Gpa which matches elastic moduli of bone (14Gpa).
■ As the elastic property of PEEK material reduces the forces created when chewing which are communicated to the implant, it has been claimed that because of the low elastic modulus of this material, the shock absorbing property of PEEK material, the stresses occurring both in abutment teeth and in the cement, interface are reduced to a minimum16. It is thought that the stress- based problems of PEEK in implantology could be overcome.
■ Various materials are used in as implant abutments, such as titanium, gold, zirconia, alumina, and glass. High-strength polymer materials such as PEEK are also recommended as abutments in many implant-supported restorations. Less bacterial biofilms are observed compared to titanium and zirconia abutments. Research has shown that PEEK can be used as an immediate definitive abutment and framework material.
■ Unmodified PEEK may have certain limitations as implant material, various surface modifications can be done to increase the bioactivity and use it as implant material. These are some of the current strategies to improve the bioactivity of PEEK14.
Physical treatment
Chemical treatment
Surface coating
Composite preparation
Physical treatment:
To improve bioactivity of PEEK several surface modifications have been tried, physical treatment like plasma modifications (such as nitrogen and oxygen plasma, methane and oxygen plasma etc) and accelerated neutral atom beam (ANAB). Plasma modification resulted into increase adhesion, proliferation and osteogenic differentiation
Chemical Treatment:
Chemical treatment like wet chemistry and sulfonation, surface coating by titanium, gold, titanium dioxide, diamond-like carbon, tert-butoxides, and hydroxyapatite have been considered. The hydroxyapatite is the most widely used material due to its biocompatibility, bioactivity, and osteo conductivity.
Surface coating: -
surface coating like deposition of aerosol, electron beam deposition, cold spray technique, radio frequency magnetron sputtering and spin coating. Bioactivity of titanium coated and uncoated implants and concluded that Ti coating of Carbon fiber PEEK screws significantly improve bone apposition and removal torque compared with uncoated Carbon fiber PEEK screws.
Composite preparation: -
This includes Carbon fiber-reinforced PEEK(CFR-PEEK), Glass-fiber-reinforced] PEEK(GFR-PEEK), Hydroxyapatite/PEEK(HA/PEEK), Strontium containing hydroxyapatite/PEEK (SrHA/PEEK) Nanosized PEEK composites -This includes Nano- TÍ02/PEEK, Nano- Fluorine apatite PEEK and Nano-hydroxyapatite PEEK
PEEK in Fixed Prosthesis
Using CAD-CAM production methods for restorations makes it possible to construct dental prostheses chair-side21. CAD/CAM designed PMMA and composites fixed dentures have exhibited superior mechanical properties compared to traditional prepared fixed dentures20,22. PEEK is a material that may be used as an alternative to PMMA for CAD/CAM restorations. PEEK fixed partial dentures constructed using CAD/CAM technique have exhibited higher fracture resistance than the pressed granular-shaped PEEK dentures The fracture resistance of CAD/CAM milled PEEK fixed dentures is much higher than that of comparable lithium disilicate glass-ceramic, alumina, and zirconia restorations23,24. Despite the significantly low elastic moduli and hardness of PEEK, the abrasive resistance of PEEK is competitive to that of metallic alloys25.
However, no clinical studies have attempted to compare the abrasion produced by PEEK crowns on teeth to that produced by other materials such as ceramics and alloys. As yet, no studies have indicated whether PEEK crowns function efficiently with the dentin and enamel. Considering PEEK's abrasion resistance, mechanical attributes and adequate bonding to composites and teeth, a PEEK fixed partial denture would be expected to have an acceptable survival rate.
Processing Of Peek for Denture Frame Works and Fixed Partial Dentures
They can be manufactured by following processes:
Injection moulding.
CAD/CAM manufacturing.
-
3D printing technology.
Injection Moulding process: -
■ The injection molding system is one of the most commonly used processing technologies in the plastics industry, and the temperature of the mold is an important factor in determining the final quality of the injected product [Figure-3].
■ In semi-crystalline materials such as PEEK, the mold temperature is an important factor in determining the parameters of the injected product for performance. PEEK demonstrates greater strength than many metals on a per mass basis.
■ Injection moulding can be an economical method for manufacturing polymer components. Injection moulding was performed in a commercially available injection moulding machine using material made from high density polyethylene thermoplastic.

- Moulded wax frame work
The PEEK molten material was injected within 60 seconds into the mould cavity with pressing pressure of 150 MPa. The cooling time for the injected PEEK polymer was 5 to 6 hours. The injected moulds were left overnight to allow slow bench-cooling to room temperature. The flask was then de-flasked and the specimens were cut from the sprues.
3D Printing technology
Additive manufacturing is used to create patient- specific 3D dentistry implants, denture, tools and devices as per patient match with the help of 3D printing technologies, 3D scanners and support software. Fused deposition modeling - FDM (or fused filament fabrication - FFF) methods are used for 3D Printing with PEEK filaments. Nozzle temperature: 360-400 degrees Celsius Heated bed: 120 degrees Celsius.
Advantages of 3D Printing technology: -
Enhanced performance
Weight reduction
Less wastage
Greater design freedom.
CAD/CAM manufacturing
fourteen high-performance polymer dental facets (PEEK) were developed using computer-aided design (CAD) software [Figure-4], then milled using a computer-assisted machine (CAM). PEEK granules by vacuum pressing or CAD-CAM milling are used as a framework for long span FPD which is finally layered with a nanocomposite. As PEEK is lighter, it may be a suitable alternative to chrome-cobalt ceramics. Furthermore, it does not corrode when in contact with other metals in the mouth. The resistance to breakage of PEEK fixed prostheses ground with CAD-CAM is higher than that of lithium disilicate glass-ceramic, aluminum, and zirconium.
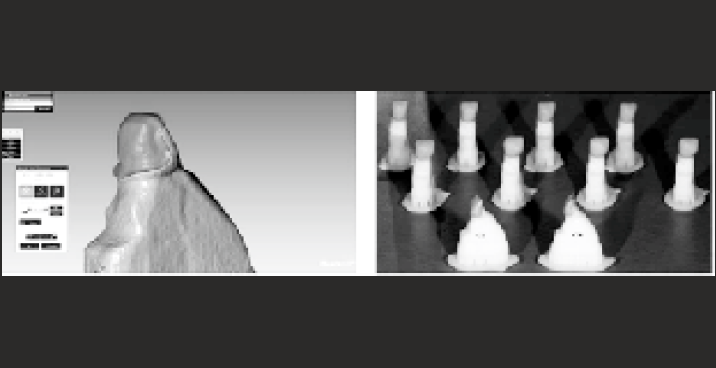
- computer-aided design and fabrication of PEEK veneers.
PEEK in Removable Prosthesis
Dentures could be constructed using PEEK CAD/CAM7. Denture clasps made of PEEK may have lower retentive forces compared to Co-Cr clasps25 However, the clasp dimensions and testing conditions may not represent the genuine clinical situations. In addition, an application of PEEK in a removable dental prosthesis was reported that successfully used PEEK material as an alternative material to metals and acrylic resins26. Yet, more studies are needed to evaluate the efficacy of PEEK prostheses compared to conventional metal and acrylic prostheses, as few published clinical studies or systematic reviews have focused on the use of PEEK as a denture material. Nevertheless, given the superior mechanical and biological properties of PEEK, dentures constructed from this polymer could well be routinely constructed in the near future. The low weight and the absence of allergy are assets boasting the merits of using PEEK (Bio-HP) as an alternative to chromium-cobalt in the realization of partial denture26 has carried out in the same patient a PAPC in chromium -cobalt and a second PEEK, the weight of the latter is 27.5% smaller than that of the first [Figure-5], even if the configuration is modified (cobalt chrome lingual bar against a lingual PEEK headband) stresses that PEEK partial dentures compared to acetal ones require a biomechanically balanced design to ensure periodontal durability30.
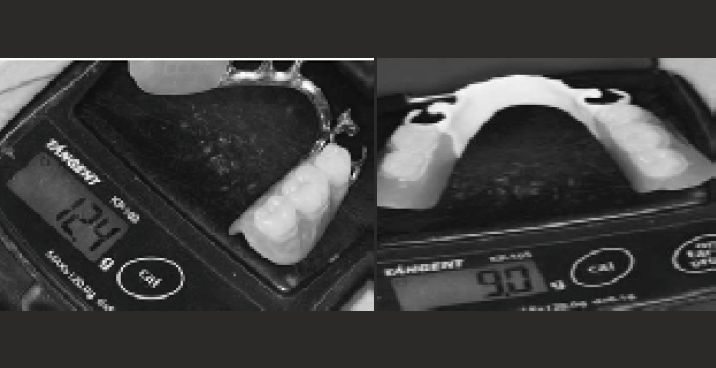
- comparison of the weight of two partial dentures in cobalt-chromium and that in PEEK.
comparison of the weight of two partial dentures in cobalt-chromium and that in PEEK.
PEEK in maxillofacial prosthesis
Due to its biocompatibility and its lightweight, the PEEK could be used as a maxillofacial prosthesis obturator. Costa-Paulau28 reports a clinical case where, in front of a loss of Centro-maxillary substance with sinus communication, a hollow shutter (0.5mm thick) could be designed numerically and machined via a 5-axis milling machine, the PEEK's lack of adhesion to the resin could be bypassed by the creation of a retention box in the shutter followed by micro abrasion by silica particles, the appointments confirm the good maturation of peri-prosthetic soft tissue, the patient reports comfort due to the lightness of the material [Figure-6].
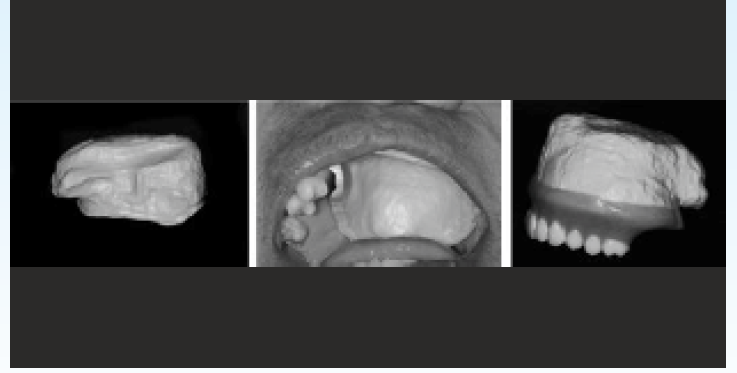
- Maxillary obturator designed in PEEK
Limitation of PEEK
However, PEEK is biologically inert, preventing good bonding with surrounding bone tissue when it is implanted in vivo. Surface modification and composite preparation are two main strategies to improve the bioactivity of PEEK32.
For surface modification, including surface chemical treatment, physical treatment, and surface coating, the stability of the modified surface will be the key issue requiring further investigation32.
Manufacturing PEEK frameworks is technique sensitive and equipment oriented, therefore is costly as compared to metallic frameworks. For the preparation of bioactive PEEK composites, the main challenge is to keep the excellent mechanical properties of PEEK when impregnating bioactive materials34.
The development of PEEK composites containing nano-sized bioactive materials may provide an effective way to obtain both mechanical and biological benefits34.
CONCLUSION:
This article reviews various properties of PEEK material, its applicability as implant material and its other applications in prosthodontics. PEEK material is a modern material attracting interest for use in dentistry. Due to the elasticity modulus which is close to that of bone and dentin, there is increasing use of the material in implantology. It can be considered that increasing the bonding of the material with acrylic and composite resins and developing the osteointegration properties will further increase dental applications.
Due to the superior mechanical and biological properties of PEEK material, it can be considered that in the future, prostheses made from polymer will have a place in routine applications.
However, still there is a vast scope for future researches and works for the innovation and development of the techniques to make it more feasible and accessible, to ensure unhampered use of PEEK and PEEK based composites to benefit the society.
Conflict of Interest:
No conflict of interest.
REFERENCES:
- Polyether-Ether-Ketone (PEEK) and its Application in Prosthodontics: A Review. South Asian Res J Oral Dent Sci. 2021;3(3):60-64.
- [Google Scholar]
- Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J Prosthodon Res. 2016;60(1):12-9.
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis, characterization and study of the thermal properties of new polyarylene ethers. Polymer. 1992;33(9):1976-81.
- [CrossRef] [Google Scholar]
- Composite technology for total hip arthroplasty. Clin Orthop Relat Res. 1988;235:224-36.
- [CrossRef] [Google Scholar]
- Stress shielding and fatigue limits of poly-ether-ether-ketone dental implants. J Biomed Mater Res Part B: Appl Biomater. 2012;100(4):1044-52.
- [CrossRef] [PubMed] [Google Scholar]
- PEEK biomaterial handbook 2012:2.
- Advantages of PEEK dental prosthetic frameworks over metal a clinical case review. Invibio Biomaterial Solutions: Dental Solutions file:///C:/Users/amin/Downloads/DentalPolymersMetals105.p df
- [Google Scholar]
- High-performance polymers and their potential application as medical and oral implant materials: a review. Implant Dent. 2015;24(4):448-57.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the stress distribution in CFR-PEEK dental implants by the three-dimensional finite element method. J Mater Sci Mater Med. 2010;21(7):2079-85.
- [CrossRef] [PubMed] [Google Scholar]
- A review of PEEK polymer's properties and its use in prosthodontics. J Stomatologia Baltic and Maxillofac. 2017;19:19-23.
- [Google Scholar]
- Influence of PEEK surface modification on surface properties and bond strength to veneering resin composites. J Adhes Dent. 2014;16:383-92.
- [Google Scholar]
- Influence of surface conditioning on bonding to polyetheretherketon (PEEK) De Mater. 2012;8:1280-83.
- [CrossRef] [PubMed] [Google Scholar]
- PEEK surface treatment effects on tensile bond strength to veneering resins. JProsthet Dent. 2014;112:1278-88.
- [CrossRef] [PubMed] [Google Scholar]
- Polyetheretherketone-a suitable material for fixed dental prostheses? J Biomed Mater Res Part B: Appl Biomater. 2013;101:1209-16.
- [CrossRef] [PubMed] [Google Scholar]
- Polyetheretherketone-cytotoxicity and mutagenicity in vitro. . 2002;23(8):1749-1759.
- [CrossRef] [PubMed] [Google Scholar]
- Titanium Implants Permanently Penetrating Human Skin. Scandinavian Journal of Plastic and Reconstructive Surgery and Hand Surgery. 1982;16(1):17-21.
- [CrossRef] [PubMed] [Google Scholar]
- Retrospective Evaluation of Crestal Bone Changes Around Implants with Reduced Abutment Diameter Placed Non-Submerged and at Subcrestal Positions: The Effect of Bone Grafting at Implant Placement. Journal of Periodontology. 2011;82(2):234-242.
- [CrossRef] [PubMed] [Google Scholar]
- Biofilm formation on the surface of modern implant abutment materials. Clin Oral Implants Res. 2015;26(11):1297-301. Epub 2014 Jul 24
- [CrossRef] [PubMed] [Google Scholar]
- Fracture strength of temporary fixed partial dentures: CAD/CAM versus directly fabricated restorations. . 2011;27(4):339-347.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical fit of all-ceramic three-unit fixed partial dentures, generated with three different CAD/CAM systems. Eur J Oral Sci. 2005;113(2):174-9.
- [CrossRef] [PubMed] [Google Scholar]
- Load-bearing capacity of CAD/CAM milled polymeric three-unit fixed dental prostheses: Effect of aging regimens. 2012. Clin Oral Invest. 16:1669-1677.
- [CrossRef] [PubMed] [Google Scholar]
- Digital dentistry: an overview of recent developments for CAD/CAM generated restorations. BDJ. 2008;204(9):505-511.
- [CrossRef] [PubMed] [Google Scholar]
- Fracture force of tooth-tooth- and implant-tooth-supported all-ceramic fixed partial dentures using titanium vs. customised zirconia implant abutments. . 2008;19(10):1049-1053.
- [CrossRef] [PubMed] [Google Scholar]
- Property maps for abrasion resistance of materials. . 2007;55(18):6365-6371.
- [CrossRef] [PubMed] [Google Scholar]
- Retentive forces and fatigue resistance of thermoplastic resin clasps. . 2012;28(3):273-278.
- [CrossRef] [PubMed] [Google Scholar]
- The Use of a Modified Poly-Ether-Ether-Ketone (PEEK) as an Alternative Framework Material for Removable Dental Prostheses. A Clinical Report. Journal of Prosthodontics, (), n/a-n/a 2015
- [CrossRef] [PubMed] [Google Scholar]
- Use of a Polyetheretherketone Clasp Retainer for Removable Partial Denture: A Case Report. Dent J (Basel). 2019;7(1):4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of pol-yetheretherketone in the fabrication of a maxillary obturator prosthesis: a clinical report. J. Prosthet. Dent. 2014;112:680-682.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of removable partial dentures on periodontal health of abutment and non-abutment teeth. J Periodontol. 2002;73(2):137-44.
- [CrossRef] [PubMed] [Google Scholar]
- PEEK vs Cr-Co : comparaison de deux types de châssis de prothèse amovible partielle. Strat. Proth. 2016;16(3):205-215.
- [Google Scholar]
- THE APPLICATIONS OF POLYETHERETHER-KETONE (PEEK) IN DENTISTRY: SYSTEMATIC REVIEW. American Journal of Innovative Research and Applied Sciences. 2020;10(2):192-200.
- [Google Scholar]
- Impact of Milled Peek Versus Conventional Metallic Removable Partial Denture Frameworks on the Abutment Teeth in Distal Extension Bases. A Randomized Clinical Trial. Int J Dent & Oral Heal. 2019;5(3):20-26.
- [Google Scholar]
- Areas for use of PEEK material in dentistry. Int Dent Res. 2018;8(2):84-92.
- [CrossRef] [Google Scholar]







